Vinyl Carbon Nmr

Simulate and predict nmr spectra directly from your webbrowser using standard html5.
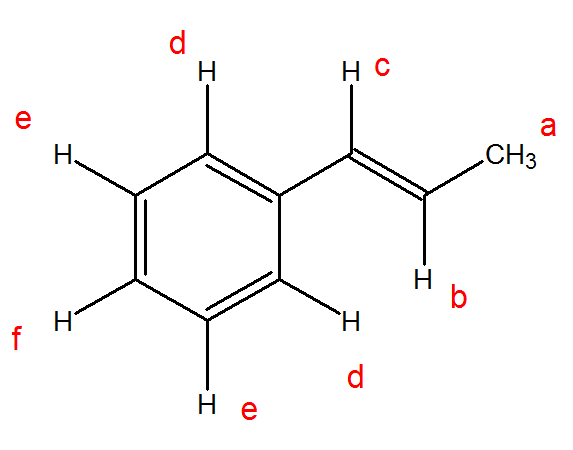
Vinyl carbon nmr. These are organic compounds containing an ethenylbenzene moiety. Second order effect like ab abx aa xx can be simulated as well. Carbonic acid cyclic vinylene ester. This is also known as 3 buten 2 one amongst many other things here is the structure for the compound.
You can also simulate 13c 1h as well as 2d spectra like cosy hsqc hmbc. 4 vinylphenol also known as p hydroxystyrene belongs to the class of organic compounds known as styrenes. 4 vinylphenol participates in a. Predict 13c carbon nmr spectra directly from your webbrowser using standard html5.
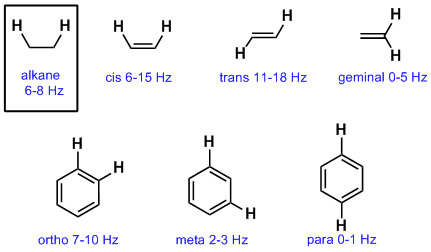
Vinyl acetate is used to make other industrial chemicals. Three deuterated species of vinyl chloride were used to facilitate the analysis and to obtain all the c 13 h couplings in this molecule. 4 vinylphenol exists as a solid slightly soluble in water and a very weakly acidic compound based on its pka. Vinyl aromatics nitriles rocr 3 arcr 2 h alkyne r 3coh o 1h 2nmr shift ranges δ ppm vinyl r 3c f r 3c clrc i r 3c br rccr 3 o δ ppm 13c nmr shift ranges r 2nh r 2ncr h approximate nmr shift ranges note.
Carbon 13 c13 nuclear magnetic resonance most commonly known as carbon 13 nmr or 13 c nmr or sometimes simply referred to as carbon nmr is the application of nuclear magnetic resonance nmr spectroscopy to carbon it is analogous to proton nmr 1 h nmr and allows the identification of carbon atoms in an organic molecule just as proton nmr identifies hydrogen atoms. 4 vinylphenol exists in all eukaryotes ranging from yeast to humans. The peak at just under 200 is due to a carbon oxygen double bond. Vinyl chloride is primarily used to make polyvinyl chloride to manufacture plastics.
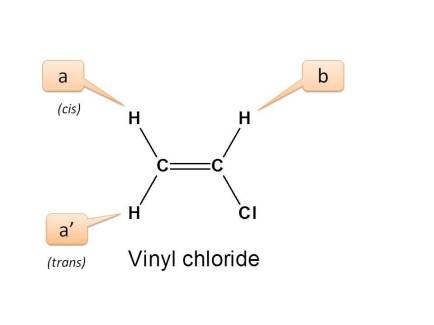
Vinyl chloride is a chlorinated hydrocarbon occurring as a colorless highly flammable gas with a mild sweet odor that may emit toxic fumes of carbon dioxide carbon monoxide hydrogen chloride and phosgene when heated to decomposition. The c 13 nmr spectrum for but 3 en 2 one. The key difference between these two structural components is the number of carbon and hydrogen atoms. Vinyl acetate is an industrial chemical that is produced in large amounts in the united states.
Both groups own a double bond between two carbon atoms where all the other atoms are bonded through single bonds. These are typical chemical shifts. The c 13 h couplings were also obtained for the other chloroethylenes. The nmr parameters are compared for the entire series of.
It is a clear colorless liquid with a sweet fruity smell. The nmr spectra of vinyl chloride the three dichloroethylenes and trichloroethylene have been obtained and analyzed. Key difference allyl vs vinyl both allyl and vinyl groups have slightly similar structures with a small variation. Allyl groups have three carbon atoms and five hydrogen atoms.
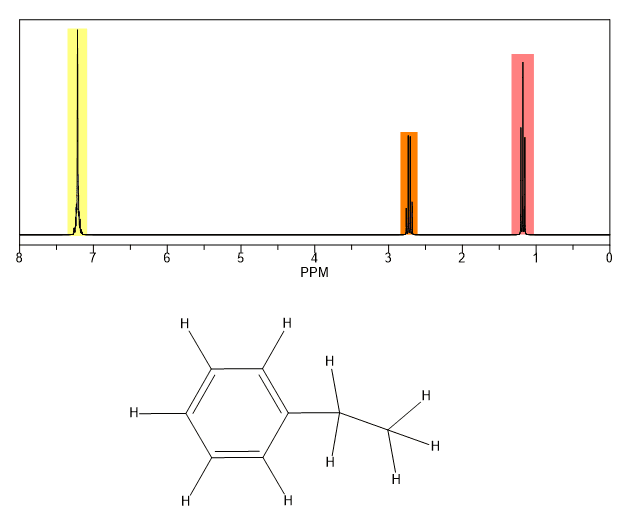
You can pick out all the peaks in this compound using the simplified table above.